Website :
https://www.biomedicale.parisdescartes.fr/combines
Contact :
combines@parisdescartes.fr
Adenohypophysis Project
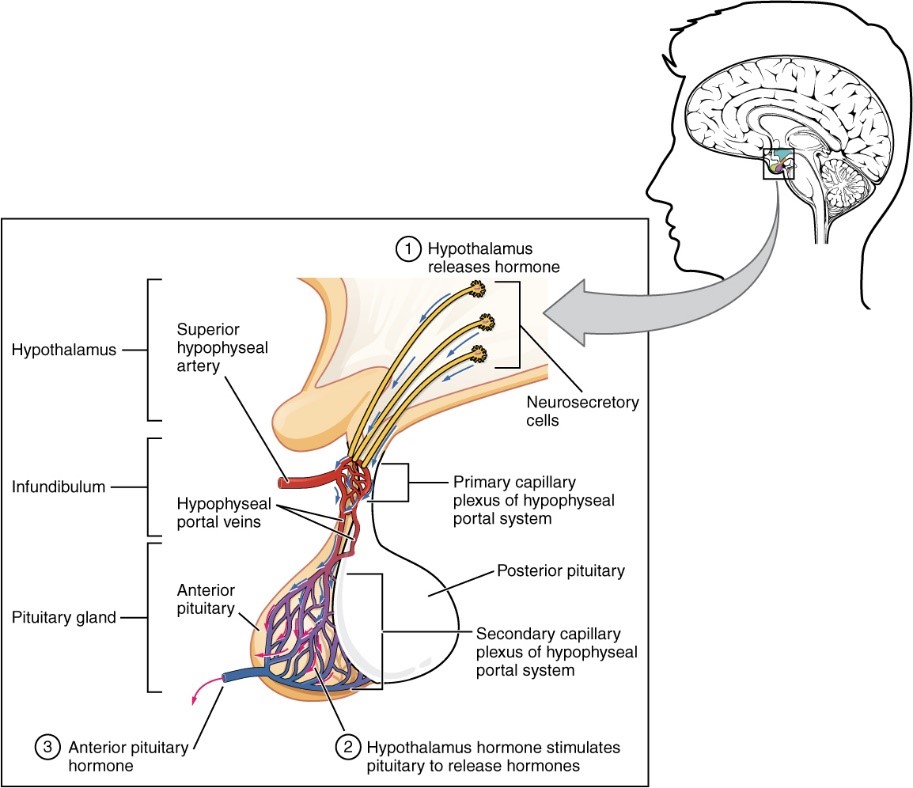
The Hypophysis (or Pituitary Gland) is an Endocrine Gland localised in the anterior part of the Brain where it is linked to the Hypothalamus. The Anterior lobe, called Adenohypophysis has many roles among others with 5 main hormones that it produces:
- The stimulation of the Thyroiodin hormone’s secretion, essential to cell development, growth and differentiation (Thyrotropin (TSH))
- Stress Response (Adrenocorticotropin (ACTH))
- Milking, Reproductive and Behavioural functions (Gonadotrophins (FSH et LH) and Prolactin (PRL))
- Cell Growth and Division (Growth Hormone (GH))
In partnership with Imagine Institute, we decided to model the Adenohypophysis because of the major role it plays in the Neuroendocrinal System and the organism’s metabolism. Additionally, its malfunction can induce many pathologies such as adenomas, pituitary dwarfism, gigantism, Cushing disease (obesity with diabetes, asthenia and hypertension) together with many disorders such as amenorrhea, sexual disorders, infertility, impotence, hypotension etc.
Only two models of pituitary organoids have been published during the past 10 years. The development of such an organoid represents a major challenge in the field of regenerative medicine, fundamental and pharmacological research.
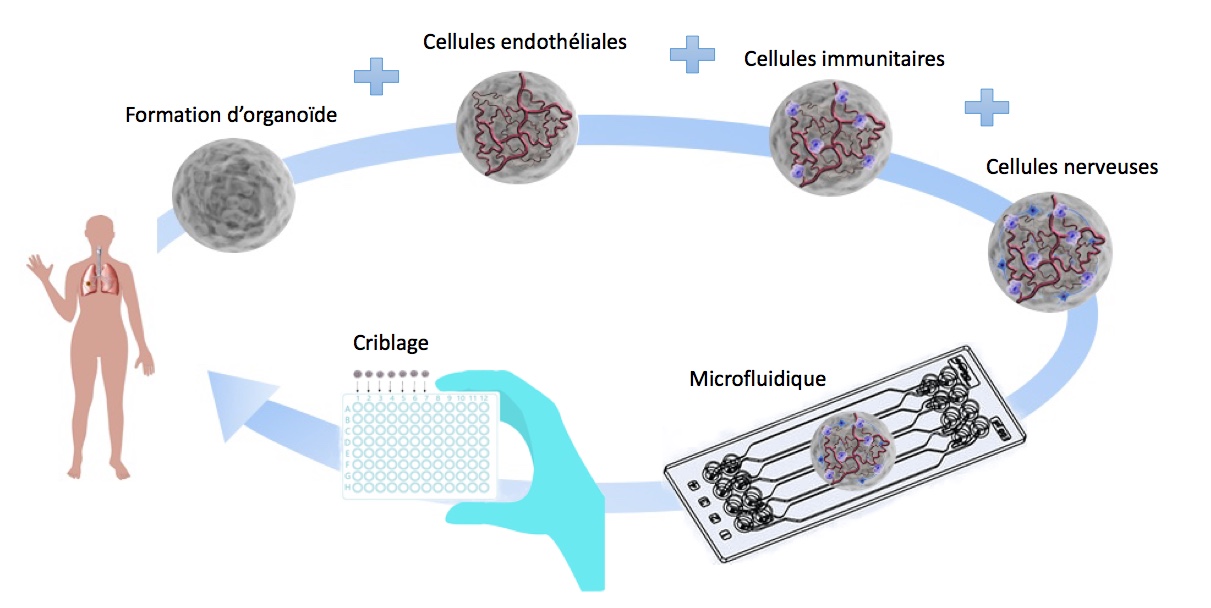
To create this Organoid, we will reproduce the Embryonic development of the Adenohypophysis by differentiating Human Induced Pluripotent Stem Cells (IPS) cultured in suspension into Oral Ectoderm then into Cranial placodes until the final pituitary cell subtypes. Our goal will be to reproduce these steps in an encapsulation microfluidic device to improve the reproductivity, reduce material costs and facilitate our experiments.
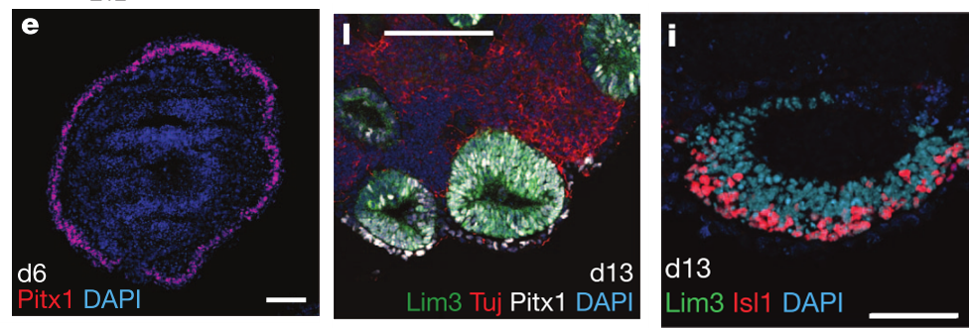
Figure 1. e. Neurectoderm formation (Day 6) l. Vesicles formation (Day 13) i. Rathke Pouch formation from the vesicles (Day 13) (Suga et al, 2011)
Retina Project
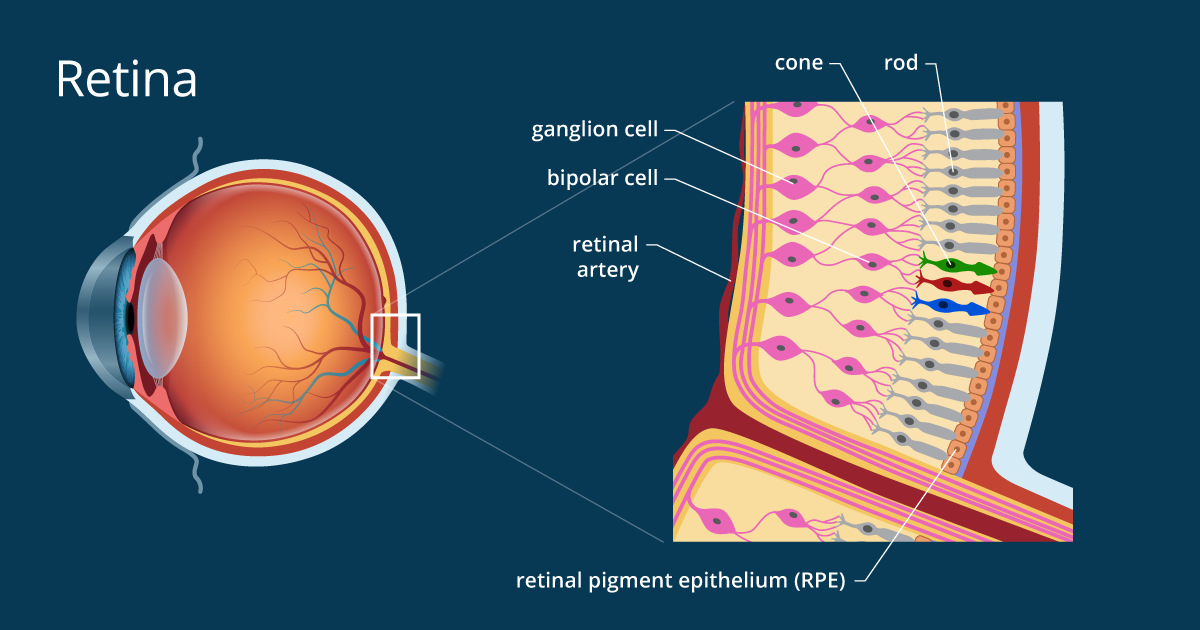
The retina is the innermost tissue and photosensitive epithelium of the eye. It is composed of two layers of different embryological origin, the nerve retina responsible for the absorption of the light signal and the pigmentary retina ensuring the conversion of the light into a bioelectric signal, transmitted mainly to the visual cortex by the optic nerve.
In partnership with Pasteur Institute, we have chosen to model the retina for its major medical issues in an aging society whose prevalence of retinal pathologies is gradually increasing. More than 170 million people are now affected by age-related macular degeneration (DMA) (Pennington & DeAngelis, 2016), a progressive deterioration of the area responsible for maximum visual acuity inducing a permanent spot in the centre of the field visual, frequently occurring in 65 years old people.
In recent years, several models of retinal organoids with different levels of complexity have been developed, with a significant structural and functional variability within and between models. They reveal some difficulties in terms of yield, functionality (especially on photoreceptors) including the presence of non-retinal structures.
Our model aims to improve the regularity, reproducibility and functionality of the retinal Organoid. Thus, to create this Organoid, we will reproduce the steps of Embryonic development of the retina by differentiating murine embryonic stem cells and induced human pluripotent cells (IPS). They will be cultured in a microfluidic system, to allow miniaturization and automation of cell culture procedures, enabling us to fulfil our objective of optimised reproducibility.
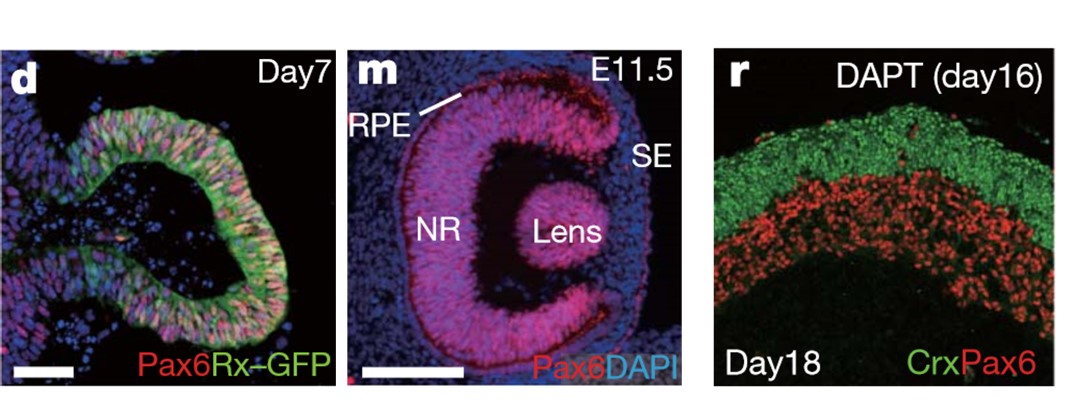
Figure 2 d.Formation of the vesicle (Day 7) m.Formation of an optic-cup-like structure (Day 16), r.Stratified neural retina tissues (Day 11.5) (Eiraku et al, 2011)
Sponsors

Members

Julie Jardon, Camille Brouillon, Guillaume Mondon, Nessim Richard

 Khaled ALASAAD ALWEKYAN
Khaled ALASAAD ALWEKYAN Benjamin LEVY
Benjamin LEVY Amad KAYTOUE
Amad KAYTOUE Amine HALIMI
Amine HALIMI